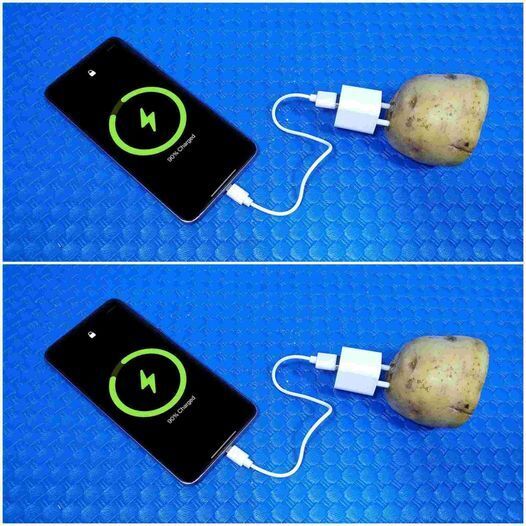
You might have heard the fun fact that you can generate electricity using a potato. It sounds like science fiction, but it’s based on real chemistry! Let’s break down how it works and whether it can actually charge something like your phone.
How Does a Potato Produce Electricity?
Potatoes contain electrolytes — natural salts and water — which allow them to act like a basic battery. But the potato itself isn’t the power source; instead, it helps conduct electricity between two different types of metals.
Here’s the basic idea:
- Two metal electrodes are inserted into the potato:
- Copper (like a penny or copper wire)
- Zinc (like a galvanized nail)
- A chemical reaction occurs between the metals and the electrolytes in the potato, creating a flow of electrons (electricity).
What You Need:
- 1 large potato (boiled briefly increases conductivity but raw works too)
- 1 copper piece (coin, wire, or strip)
- 1 zinc piece (galvanized nail or screw)
- Two wires with alligator clips
- Small LED light or low-power device (for demonstration)
- Multiple potatoes if needed
Steps:
- Insert the copper and zinc pieces into opposite ends of the potato, keeping them a few centimeters apart.
- Connect one wire to the copper and one to the zinc using the alligator clips.
- Attach the other ends of the wires to a small device (like an LED light).
The potato battery should create a small current, enough to light up an LED.
Can You Charge Your Phone with a Potato?
Technically, no — not directly. A single potato generates about 0.8 to 1 volt of electricity, while most smartphones require 5 volts and higher current to charge. Even connecting several potatoes in series (linking them together to increase voltage) produces only a small, unstable current, far below what a phone battery needs.
What Is It Good For?
- Educational experiments
- Powering tiny devices like LEDs or digital clocks
- Fun science projects
Conclusion:
While charging your phone with a potato is more myth than reality, it’s a fascinating demonstration of natural electricity and chemistry at work. It’s a great hands-on project to show how energy can be generated in unexpected ways!